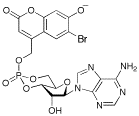
Bhc-cAMP
(6-Bromo-7-hydroxycoumarin-4-yl)methyl adenosine 3',5'-cyclic monophosphate, axial isomer, Bhc-cAMP (ax); equatorial isomer, Bhc-cAMP (eq)
| Structure @
| Absorption Spectrum
| |||||
| Photochemical and Photophysical Properties | ||||||
| substrate | Ιa(Γ)b | Σdisc | Σappd | ΣΓe | sf | t1/2g |
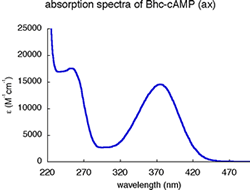
| Bhc-cAMP (ax) | 375 (14,550) | 0.081 | 0.100 | 1 120 | 440 | 260 |
| Bhc-cAMP (eq) | 371 (17,000) | 0.107 | 490 | 90 | ||
| [a] Absorption maximum (nm) measured in K-MOPS (pH 7.2). [b] Molar absorptivity (M-1cm-1). [c] Quantum yields for the disappearance of the starting materials upon irradiation (350 nm). Samples (10 uM) in K-MOPS (pH 7.2) were photolysed with two RPR 350 nm lamps. [d] Quantum yields for the appearance of the cyclic nucleotides upon irradiation (350 nm). [e] The product of quantum yields for the disappearance and molar absorptivity at 350 nm. [f] Solubility (uM) in K-MOPS (pH 7.2) containing 1% DMSO. [g] Half-life (h) in the dark. Samples (10 uM) in K-MOPS (pH 7.2) were placed in the dark at room temperature. | ||||||
Spectroscopic Properties
(axial isomer): 31P NMR d (DMSO-d6) -4.98; 1H NMR d (DMSO-d6) 8.33 (1H, s), 8.10 (1H, s), 7.55 (1H, s), 7.36 (2H, s), 6.41 (1H, s), 6.04 (2H, s), 5.81 (1H, s), 5.35 (1H, m), 5.28 (2H, d, J =7 Hz), 4.65-4.55 (2H, m), 4.34 (1H, ddd, J = 10, 10 and 2 Hz), 4.18-4.17 (2H, m). (equatorial isomer): 31P NMR d (DMSO-d6) -3.62; 1H NMR d (DMSO-d6) 8.39 (1H, s), 8.18 (1H, s), 7.58 (1H, s), 7.37 (2H, s), 6.37 (1H, s), 6.07 (2H, s), 5.80 (1H, s); HRMS (FAB+) Calcd for C20H18O9N5BrP: 582.0026 and 584.0006. Found: 582.0027 and 584.0010
References
synthesis
1) T. Furuta, H. Takeuchi, M. Isozaki, Y. Takahashi, M. Sugimoto, M. Kanehara, T. Watanabe, K. Noguchi, T. M. Dore, T. Kurahashi, M. Iwamura, R. Y. Tsien, Bhc-cNMPs as either water-soluble or membrane-permeant photo-releasable cyclic nucleotides for both one and two-photon excitation, ChemBioChem, 5, 1119-1128 (2004).
application
1) T. Nishigaki, C. D. Wood, Y. Tatsu, N. Yumoto, T. Furuta, D. Ellias, K. Shiba, S. A. Baba, A. Darszon, A sea urchin jelly peptide induces a cGMP-mediated decrease in sperm intracellular Ca2+ before its increase, Dev. Biol. 272, 376-388 (2004).
@
¨β’νΉFMεwwΆ¨ͺqΘwΘ@ΓcυΊ
TCgΜζEΆΜ]ΪπκΨΦΆά·B
Copyright© 2004@Furuta lab Toho Univ.@All Rights Reserved.
@