
_ΜP[WOiBhc-cGMPΜ¬@ j
![]()
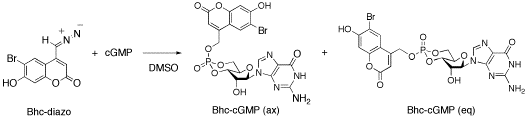
6-Bromo-7-hydroxycoumarin-4-ylmethyl guanosine cyclic 3',5'-monophosphate, Bhc-cGMP
A mixture of cGMP (17.4 mg, 50.4 mmol) and Bhc-diazo(28.9 mg, 103 mmol) in DMSO (0.5 mL) was stirred at room temperature for 76 h. The reaction mixture was directly purified by flash column chromatography (5 g of SiO2, 10% MeOH in CHCl3 then 25% MeOH in CHCl3) to yield 7.0 mg (11.7 mmol, 23% yield) of Bhc-cGMP. The axial and equatorial isomers were separated by semi-preparative reversed phase HPLC (Column: COSMOSIL 5C18 AR-II, 250 X 20, Eluent: 50% MeOH-H2O).
axial isomer: 31P NMR d (DMSO-d6) -5.06; 1H NMR d (DMSO-d6) 10.52 (1H, s), 7.90 (1H, s), 7.60 (1H, s), 6.44 (2H, s), 6.32 (1H, s), 6.19 (1H, s), 5.90 (1H, s), 5.79 (1H, s), 5.25 (2H, d, J = 7 Hz), 4.8-4.5 (2H, m), 4.2Π4.0 (4H, m), equatorial isomer: 31P NMR d (DMSO-d6) -3.90; 1H NMR d (DMSO-d6) 10.53 (1H, s), 7.94 (1H, s), 7.78 (1H, s), 6.66 (1H, s), 6.58 (2H, s), 6.27 (1H, s), 6.14 (1H, s), 5.84 (1H, s), 5.32 (2H, d, J = 7 Hz), 5.15 (1H, m), 4.70 (1H, m), 4.56 -4.40 (3H, m); HRMS (FAB+) Calcd for C20H18O10N579BrP: 597.9975. Found: 598.0023.
QlΆ£
T. Furuta, H. Takeuchi, M. Isozaki, Y. Takahashi, M. Sugimoto, M. Kanehara, T. Watanabe, K. Noguchi, T. M. Dore, T. Kurahashi, M. Iwamura, R. Y. Tsien, Bhc-cNMPs as either water-soluble or membrane-permeant photo-releasable cyclic nucleotides for both one and two-photon excitation, ChemBioChem, 5, 1119-1128 (2004).
¨β’νΉFMεwwΆ¨ͺqΘwΘ@ΓcυΊ
TCgΜζEΆΜ]ΪπκΨΦΆά·B
Copyright© 2004@Furuta lab Toho Univ.@All Rights Reserved.
@